We will call your patient within 1-2 business days to explain how to get started. We will also send you updates about your patient's Ixcela journey so that you have peace of mind they are getting the supportive care they need.
If you don't have a fax, you can simply fill out our patient referral form. We will call your patient within 1-2 business days to explain how to get started.
Refer A Patient Refer A PatientWe will also send you updates about your patient's Ixcela journey so that you have peace of mind that they are getting the supportive care they need.


Ixcela’s virtual Registered Dietitian clinic provides unique expertise in improving gut health. We accept major commercial insurance to cover Registered Dietitian visits.
Our standard procedure is to use our proprietary at-home gut health test that allows our dietitians to create highly personalized recommendations for patients, which can be paid for by HSA, unrestricted FSA, or by credit card. We also have a low-cost digital evaluation tool that uses AI and survey questions to estimate the state of the gut.
You can refer patients to Ixcela by faxing the patient's demographic data and last session note to (781) 404-8868. Another option is to complete the patient referral form. We will call your patient within 1-2 business days to talk through options and explain how to get started. We will also send you updates about your patient's Ixcela journey so that you have peace of mind that they are getting the supportive care they need.
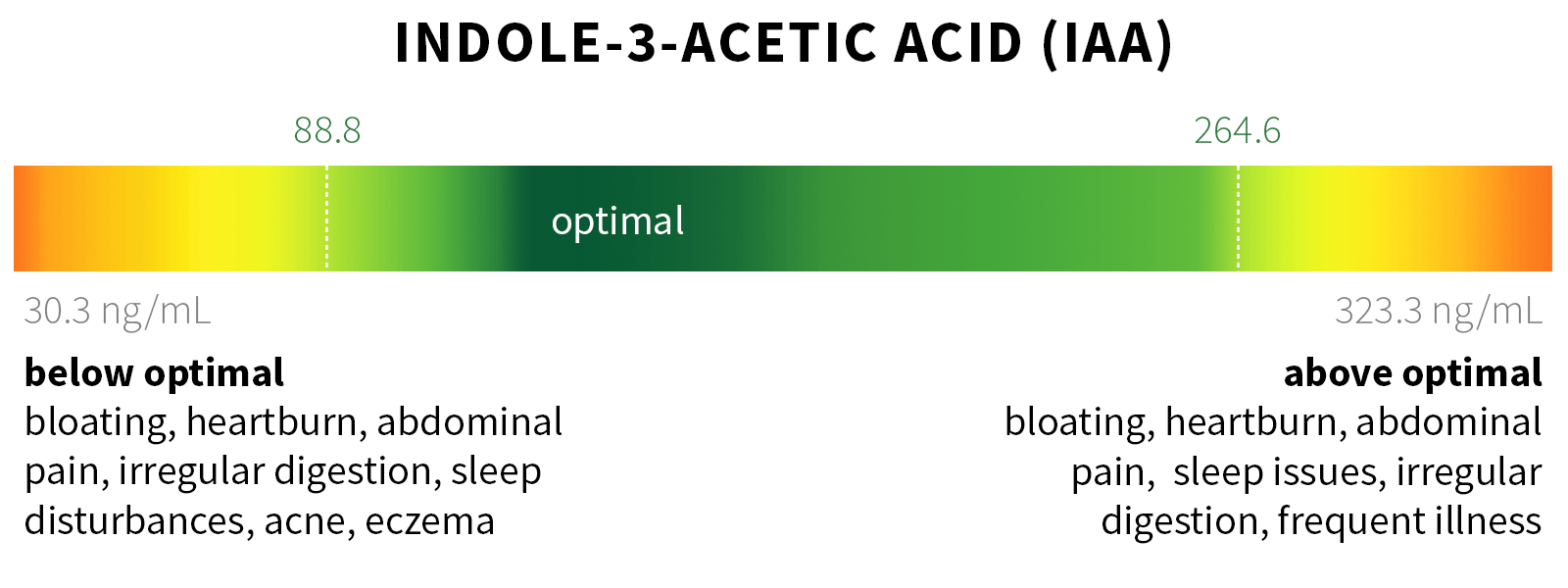
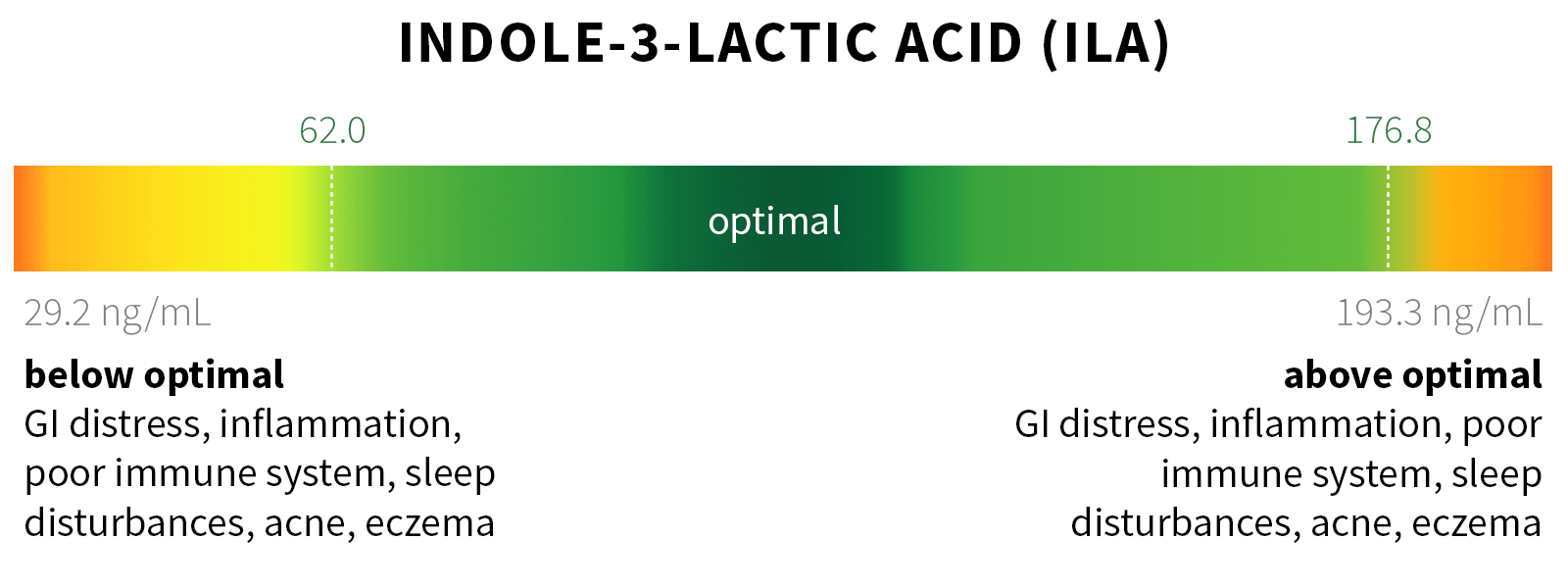
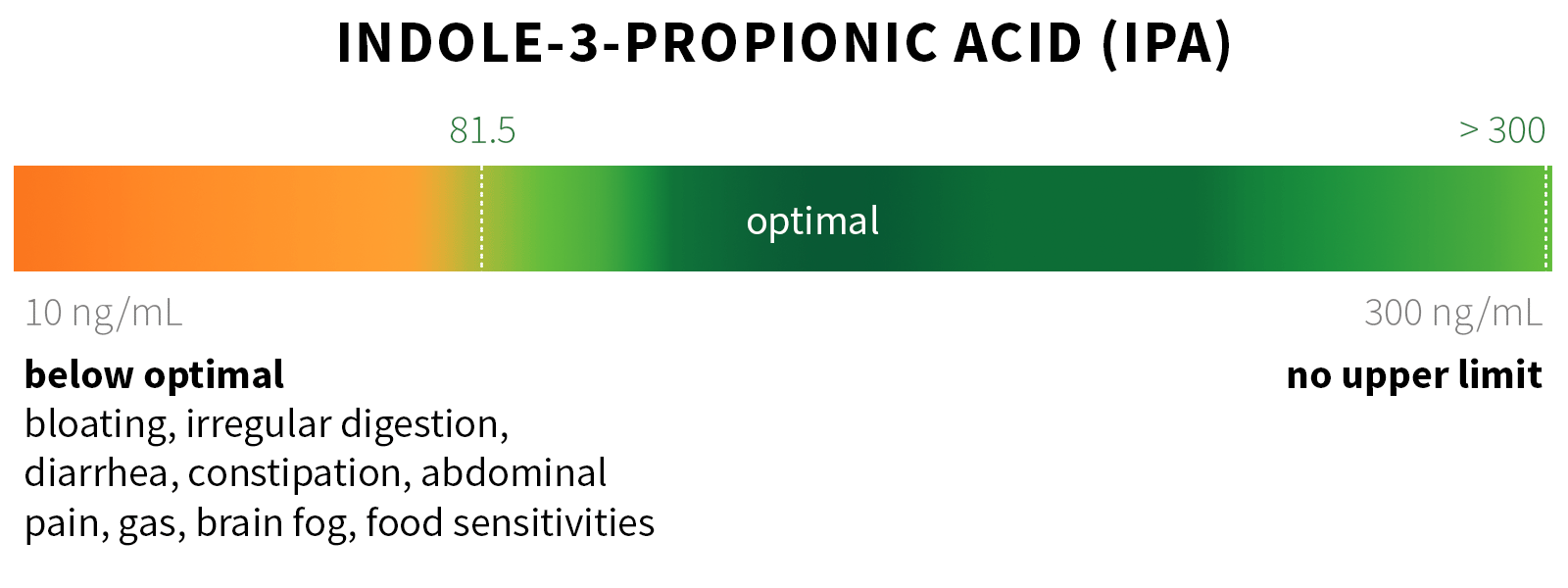
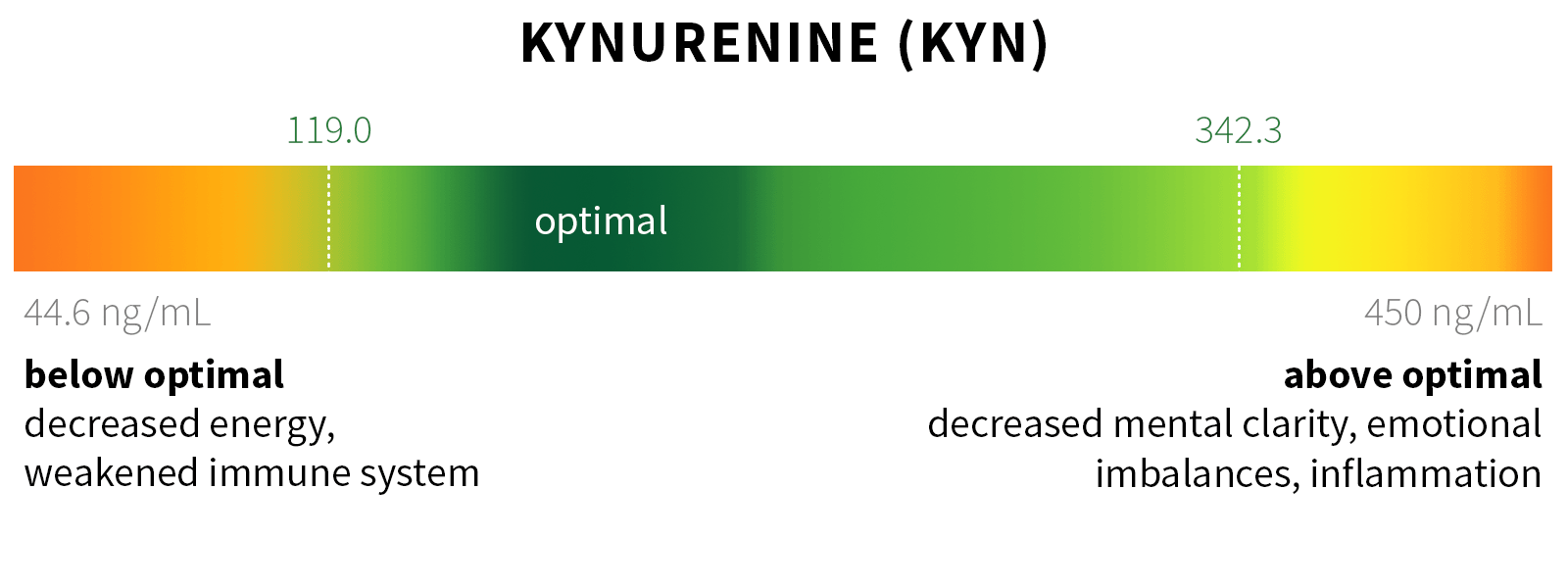
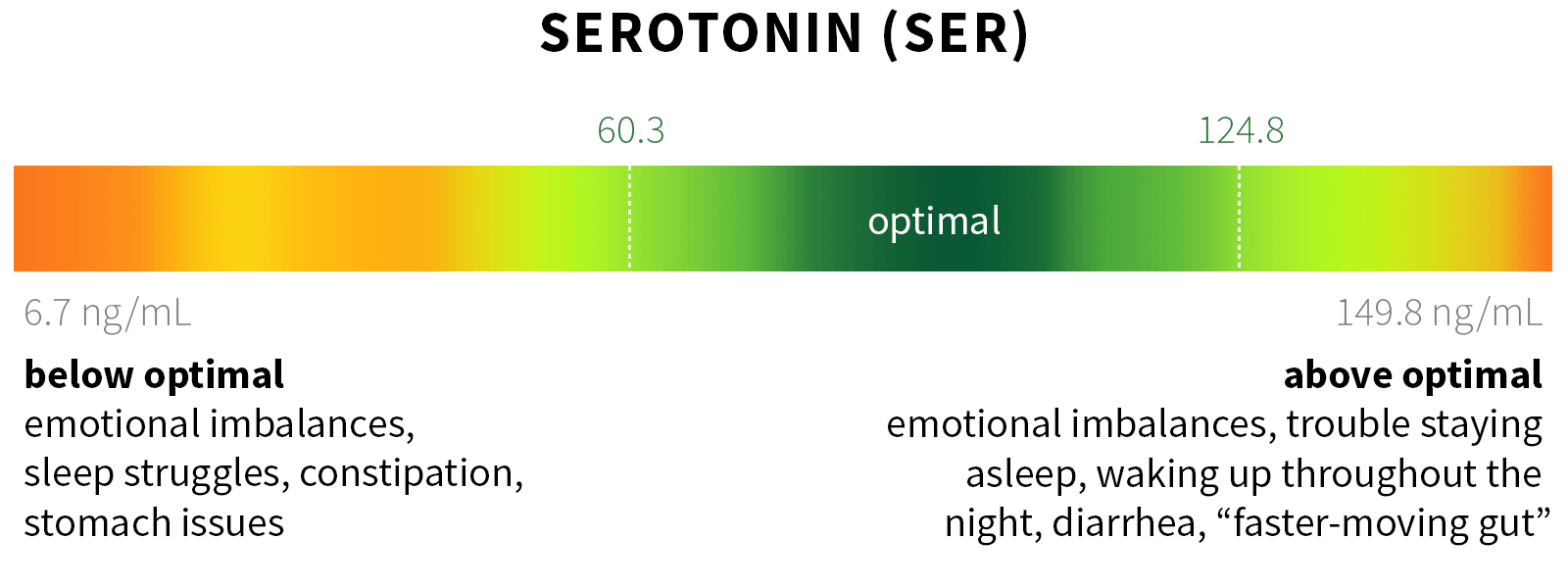
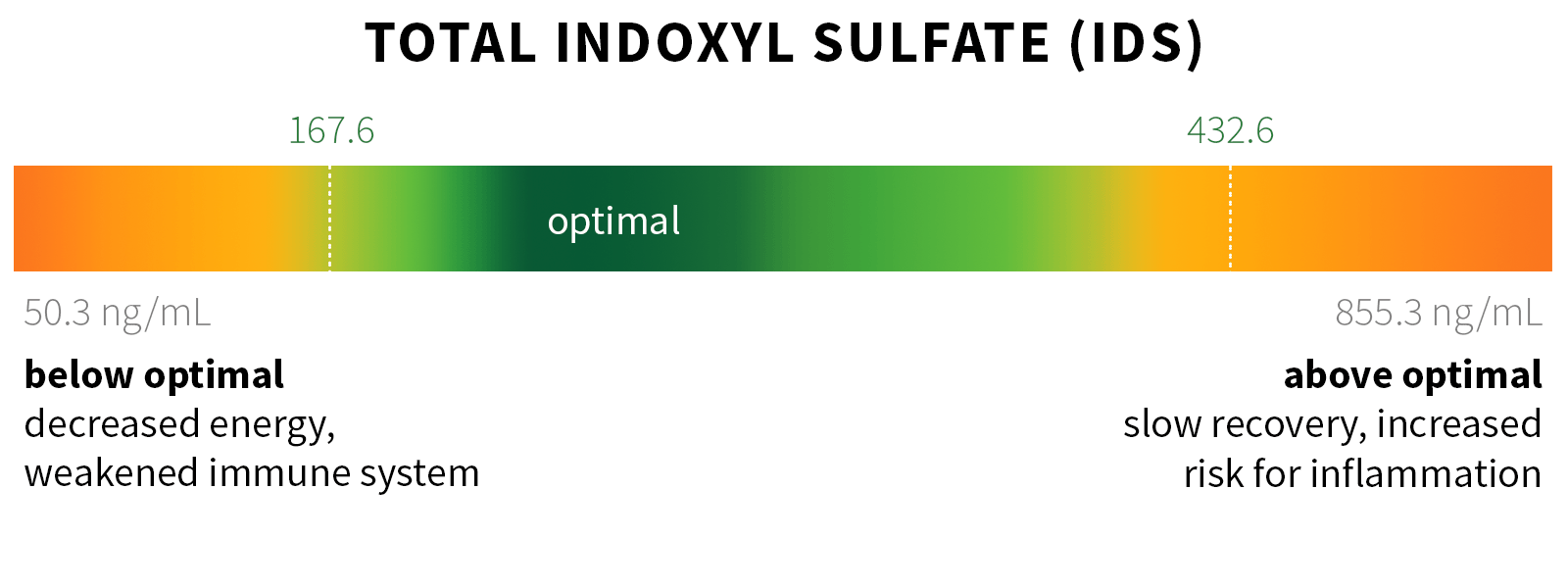
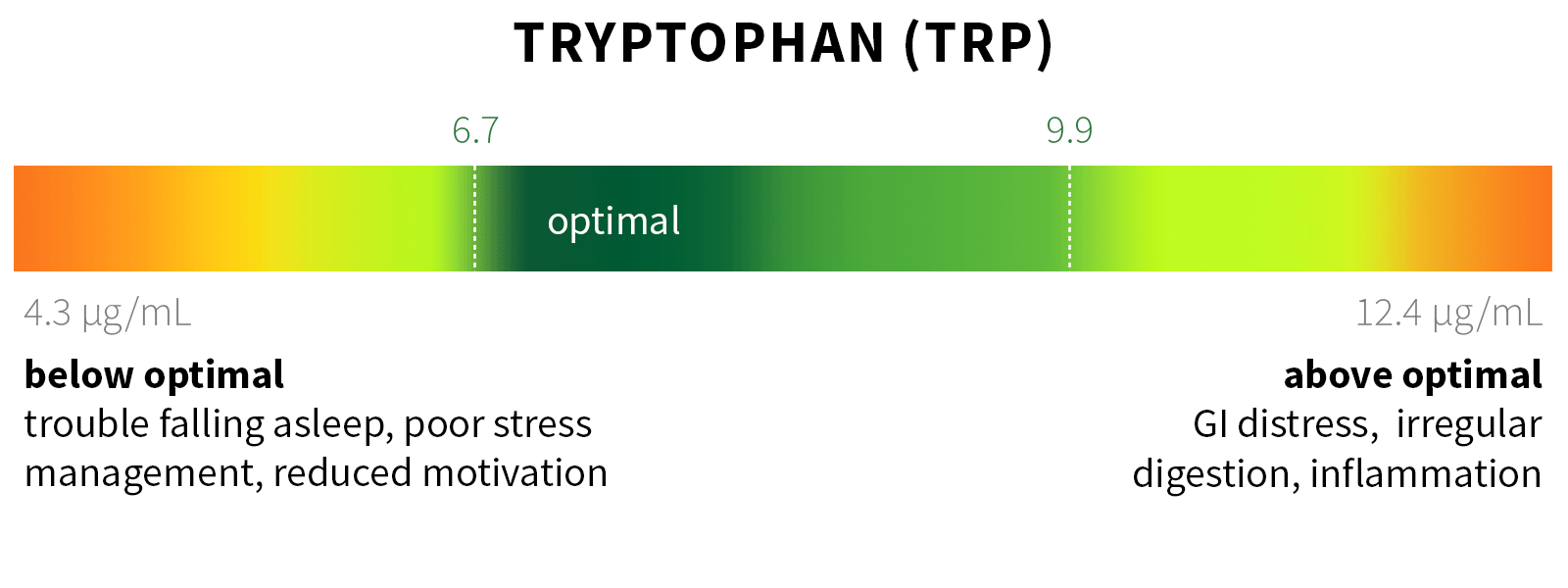
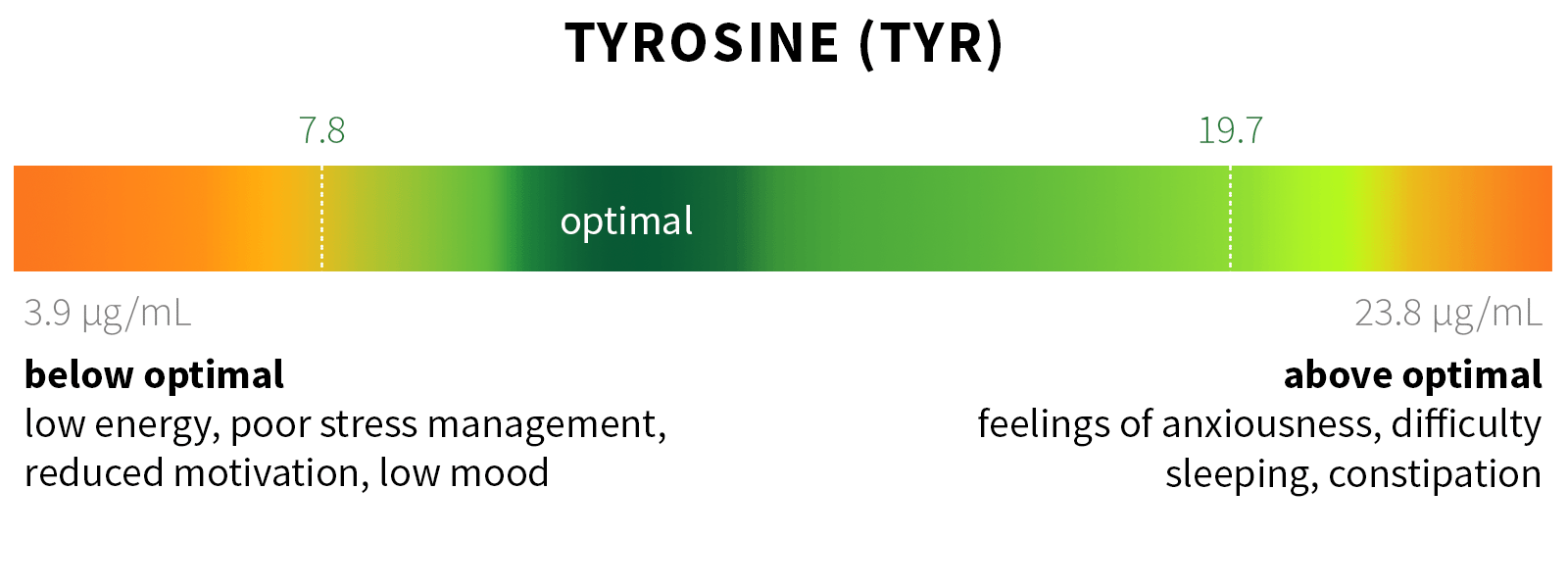
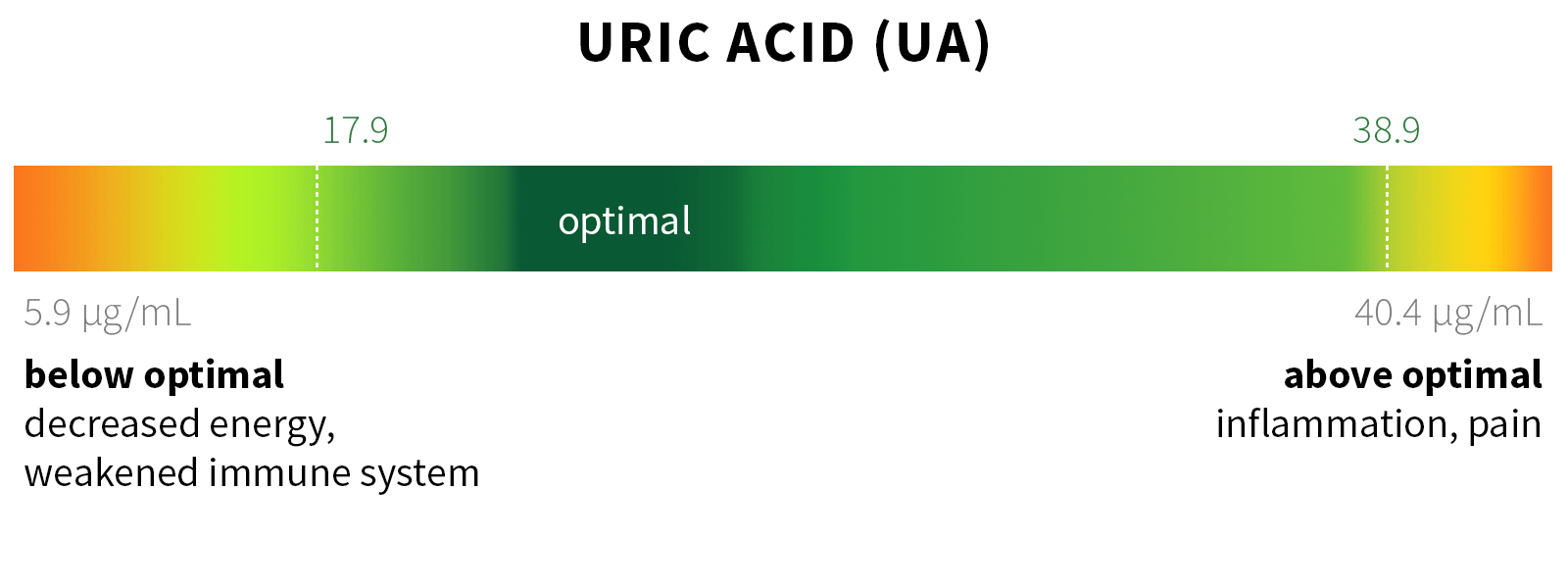
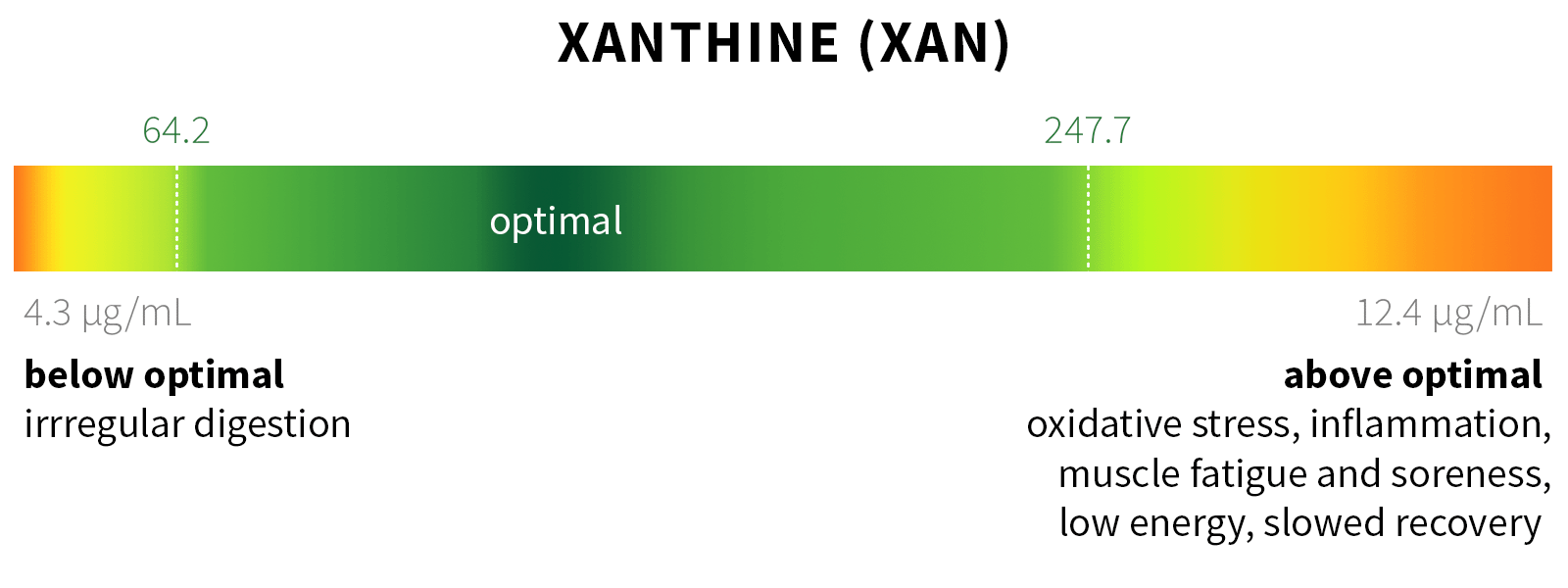
Ixcela provides supportive care services for patients who are experiencing various health challenges including:

Ixcela research comes from in-house experimentation (conducted with pharmaceutical partners like Pfizer, nonprofit organizations like NASA, and academic institutions such as Harvard, Massachusetts General Hospital, Duke, and Mayo Clinic), peer-reviewed literature, and clinical studies conducted at other laboratories around the world.