

by Lillian So Chan with Manny W Radomski, PhD
To defend against the novel COVID-19 virus (SARS-CoV-2 that causes the COVID-19 infection), we need to rally all the immune tools and troops in our body. But our most potent immune influencer is often the most ignored.
Aside from avoiding the virus, the immune system is our body’s best defense against COVID-19. If infection occurs, a strong, well-orchestrated, and well-regulated immune response is our best weapon to ensure milder symptoms and a quicker resolve.
Even when a vaccine is available, effective protection depends on the vaccine’s ability to stimulate our immunity, and the quality and duration of our immune response to it. Antibodies induced after infection or exposure recognize and inactivate COVID-19 virus. Whether these antibodies can effectively protect against reinfection and how long the protection may last also depends, at least partly, on our immune system.
On the other hand, dysregulated and excessive immune responses cause uncontrolled inflammation, tissue damage, and serious disease outcomes such as organ failure and even death.
In the past weeks, scientific journals have published an increasing number of clinical and laboratory studies highlighting the involvement of gut environment and gut microbiome in COVID-19 infection and severity.
Why and how is COVID-19 infection associated with gut and gut microbiome?
We often forget that our body’s largest immune organ is our gut. Because the gut is the most hazardous and longest border our immune system has to guard, about 70% of our immune system is located in and around our gut.
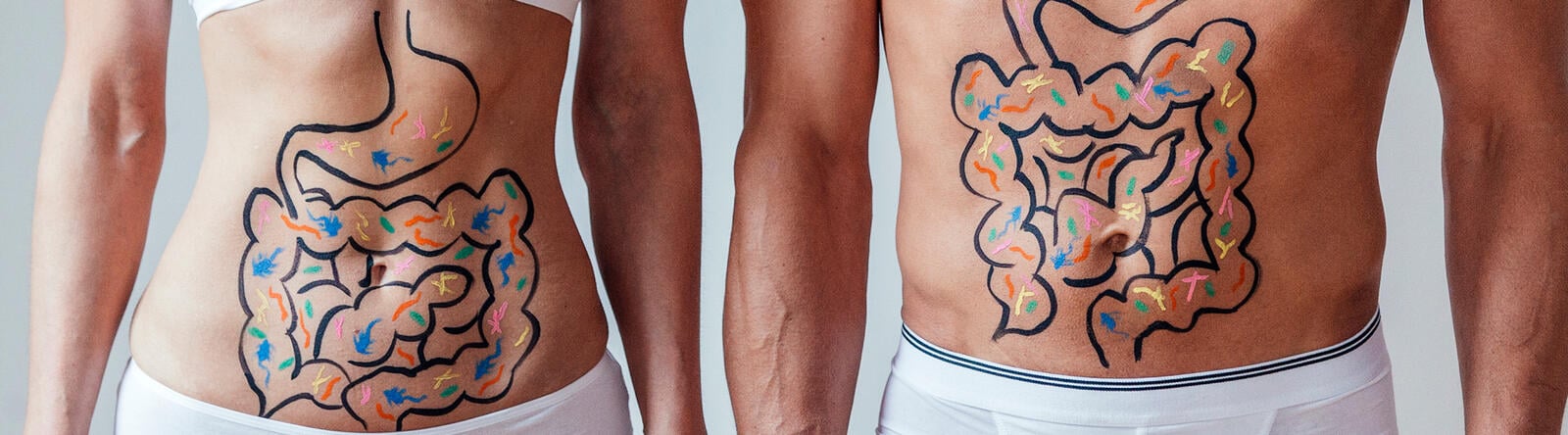
Numerous scientific studies have shown that our immune system and our gut with its microbiome influence each other to orchestrate whole-body physiology and regulate immune functions. An effective and balanced collaboration between them is essential for the development and maturation of our immunity.
Our gut microbiome is the signaling hub. It constantly integrates environmental inputs with immune and gene expression signals to regulate immune responses and homeostasis. It influences not only the gut, but also other organs throughout the body.
The gut microbiome is a potent influencer, regulator, communicator, and collaborator of our immune system, impacting its maturation, function, and regeneration. In fact, the composition and function of our gut microbiome influence the quality, strength, intensity, duration, and appropriateness of any immune reaction.
A healthy gut and gut microbiome protect, but a leaky gut and an unbalanced gut microbiome may create an inflammatory environment that the coronavirus can exploit. Inflammatory proteins (cytokines) can be amplified by more cytokines when coronavirus hits. In fact, a dysregulated, runaway immune reaction can cause more damage than the virus itself.
Previous studies indicate that the gut microbiome can boost host response to respiratory viral infections, while dysbiosis of the gut microbiome can worsen the outcome of infectious respiratory diseases.
The importance of gut microbiome on our whole-body immunity is now well established and indisputable. In the last decade, scientists have gradually discovered the surprisingly strong connection between the gut microbiome and respiratory health. This connection is called the gut microbiome-lung axis.
Lung and gut microbiota play several important roles in the development, regulation, and maintenance of healthy immune responses. Dysbiosis and subsequent dysregulation of microbiome-related immunological processes affect the onset of airway and gut diseases, their clinical characteristics, and our responses to treatment.
Over the last few years, mounting evidence shows that the gut microbiome protects against airway and lung infections, including virus infections and pneumonia. Current research is assessing the effects of selected gut microbes on improving respiratory immunity and health.
Studies also show that gut microbiome depletion and dysbiosis led to an impaired lung immune response, increased respiratory virus infection vulnerability and severity, and worsened disease outcomes. For example, researchers showed that within fifty hours of pneumonia infection, 20% of mice with depleted gut microbiome had died. In contrast, all of the infected mice with an intact gut microbiome survived.
Gut microbiome also helps to determine the effectiveness of some vaccines, including flu shots (influenza is a coronavirus). It seems that gut microbiome and gut microbial-derived signals may be necessary to induce an immune response to a flu vaccine.
Studies of the gut microbiome-lung axis may provide crucial new insights into the prevalence, etiology, and potential mechanisms of COVID-19 in the respiratory and digestive tracts. These findings will help to define prevention measures, clinical care, and treatment strategies. In the meantime, knowledge of the effects of gut microbiome on respiratory virus infections and pneumonia can inform how we may strengthen our immune defense against them.
In a recent scientific study, researchers analyzed how different components of the immune system respond to COVID-19 in the infected cells of ferrets and human patients. They characterized the gene expression (transcriptional) signature underlying COVID-19 pathology and compared it to other coronavirus infections.
They discovered that despite COVID-19 virus replication, host levels of the antiviral proteins (interferons) consistently remained abnormally low. At the same time, levels of some pro-inflammatory cytokines that generate nonspecific immune responses were significantly, abnormally high. The coupling of these two immune dynamics greatly compromises our defense and fighting capacity against COVID-19, while causing immune overreactions.
The weak antiviral response failed to prevent COVID-19 spread at early stages of the infection. But simultaneously, the inappropriate, dysregulated immune responses drive uncontrolled inflammation, causing lung injuries, organ failures, and death, as seen in severe cases of COVID-19.
The researchers indicated that their findings are consistent with clinical observations of COVID-19 pathology and data from other studies. To address these imbalanced immune dynamics, they proposed targeting immune regulation (immunomodulation) as a strategy. Clinical studies of new therapeutics could consider including microbiome and metabolite analyses to determine whether microbiome features correlate with responses to COVID-19 treatments, they suggested.
Further research to determine the viability and infectivity of COVID-19 in feces is also needed to control the spread of the virus, especially in asymptomatic carriers. More research is needed to establish direct links and the effects of gut and gut microbiome on COVID-19 infection.
In any event, taken together, there is strong evidence that a healthy gut and balanced microbiome protect against respiratory virus infections, improve our capacity to fight them, reduce risk of complications, improve our chance of better disease outcomes, and enhance certain vaccines’ effectiveness.
Our most ignored body system is actually our most important immune influencer.
If robust immune engagement and balanced immune regulation are indeed keys to protect and fight against COVID-19, strengthening our gut with its microbiome may fortify our defense against it.
Besides being a predictor of COVID-19 infection severity in patients, as shown in recent clinical studies, perhaps gut microbiome may also predict our susceptibility to the disease; the healthier the gut and the gut microbiome, the stronger the defense and the better the disease outcomes.
References on how immune system works and how gut microbiome impacts immunity

Curious about how well your gut is supporting your immune system? The Ixcela test measures how well your gut is supporting five ares of health including Gastrointestinal Fitness, Emotional Balance, Cognitive Acuity, Energetic Efficiency, and Immuno Fitness.

 |
Lillian So Chan is the founding editor of WellnessOptions, a print magazine and website, and author of the book WellnessOptions Guide to Health published by Penguin Books. With over thirty years of experience in journalism and editing, Lillian has established unique editorial directions for several award-winning publications. She has worked for Maclean’s, Canada's largest news magazine, and served as a Governor and Deputy Chairperson of the Board of Governors at the Simon Fraser University, British Columbia, Canada. |

|
He served as Scientific Advisor to the Chief of Air Staff, Defence Canada; Board Director of the Canadian Defence Research and Development Executive Committee; member on the NATO Research and Technology Agency’s Human Factors and Medicine Panel. He is the former Editor-in-Chief of the Undersea Biomedical Research Journal and serves as a referee for the Aviation, Space, and Environmental Medicine Journal. He has published on diving and aerospace medicine, human performance and protection, stress endocrinology, sleep, tropical medicine, and circadian disorders. Manny is a co-editor of WellnessOptions. |
|
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Interested in learning more about Ixcela? Learn more about Ixcela’s Internal Fitness Test to help you optimize your health.
|